Most cells in your body come with two genetic libraries; one in the nucleus, and the other inside structures called mitochondria – also known as the ‘powerhouses of the cell’.
Until now, we’ve only had a way to make changes to one.
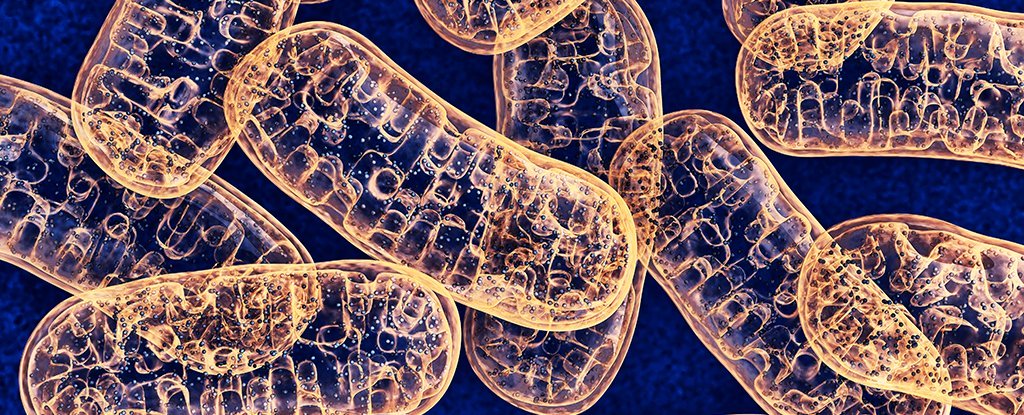
A combined effort by several research teams in the US has led to a process that could one day allow us to modify the instructions making up the cell’s ‘other’ genome, and potentially treat a range of conditions that affect how we power our bodies.
The molecular foundation of this revolutionary gene editing tool is a toxin called DddA, secreted by the bacterium Burkholderia cenocepacia to sabotage other microbes when competition over resources turns serious.
Researchers from the University of Washington have had an interest in the toxin’s talents for a while, finding it converts a nucleic acid base called cytosine into a different one commonly found in RNA, called uracil.
It’s far from the first time researchers have looked to bacterial weapons for clues on how to tweak DNA in this way. In fact, a whole family of so-called deaminase enzymes had already been put to use in genetic engineering.
Unfortunately deaminase enzymes tend to only perform their code-swapping trick on single strands of DNA.
To get around this, another research team from the Broad Institute of MIT and Harvard combined their code-swapping deaminase with CRISPR technology, which entails using an RNA template to identify the sequence and then using enzymes to unzip the strands and make changes.
That isn’t too much of a problem when you want to make edits to double strands of DNA inside something as welcoming as a cell’s nucleus. But smuggling the RNA templates through the more selective membrane of a mitochondrion isn’t quite so simple.
That’s because more than a billion years ago, mitochondria were organisms in their own right, and over time they evolved to share responsibilities with the cells they now occupy, being delegated the business of breaking down glucose for power.
While many mitochondrial genes have long since been filed away in the host’s nucleus, these tiny power units have held onto a few important sequences, which are tightly locked away behind a veil of membranes that don’t take kindly to stray bits of RNA wafting through.
Fortunately, DddA had a unique talent for making changes to both DNA strands, opening the way to ditching CRISPR – and its bulky RNA template – in favour of alternative methods for targeting the sequence you want to change.
That third piece of the puzzle came in the form of an old school genetic engineering tool called a transcription activator-like effector, or TALE.
This class of enzyme can be tailored to find specific nucleic acid codes and break them apart. Just the thing for guiding a cytosine-swapping toxin into place.
Teamed up with DddA, a specially crafted TALE enzyme can find a target sequence inside mitochondria and turn any cytosine it finds into a uracil, which will later transform into a similar DNA-specific base called thymine.
In testing, this change occurred roughly half of the time.
A fifty-fifty change might not seem like a big win, but given there were no signs of potentially disastrous changes outside of target sequences, it makes for a promising precision engineering tool.
What’s more, given there’s no other contenders for editing mitochondrial genes, it’s a landmark achievement with even this success rate.
Just as mutations in nuclear DNA can give rise to a wide variety of health conditions, mutations in the mitochondria’s genes can also be problematic, affecting anything from brain development to muscle growth, energy levels, metabolism, and immunity.
Usually (though not always) passed through the eggs down from mothers, mitochondria and any damaging mutations can be inherited through the generations. Right now the best we might be able to do is combine cells from two different mothers to remove affected mitochondria.
But with this new DddA technology, we might finally be able to create animal models that mimic a range of debilitating mitochondrial conditions in humans. And, maybe one day, even fix them inside our own bodies.






 Photographer Finds Locations Of 1960s Postcards To See How They Look Today, And The Difference Is Unbelievable
Photographer Finds Locations Of 1960s Postcards To See How They Look Today, And The Difference Is Unbelievable  Hij zet 3 IKEA kastjes tegen elkaar aan en maakt dit voor zijn vrouw…Wat een gaaf resultaat!!
Hij zet 3 IKEA kastjes tegen elkaar aan en maakt dit voor zijn vrouw…Wat een gaaf resultaat!!  Scientists Discover 512-Year-Old Shark, Which Would Be The Oldest Living Vertebrate On The Planet
Scientists Discover 512-Year-Old Shark, Which Would Be The Oldest Living Vertebrate On The Planet  Hus til salg er kun 22 kvadratmeter – men vent til du ser det indvendigt
Hus til salg er kun 22 kvadratmeter – men vent til du ser det indvendigt  Superknepet – så blir snuskiga ugnsformen som ny igen!
Superknepet – så blir snuskiga ugnsformen som ny igen!  Meteorite That Recently Fell in Somalia Turns Out to Contain Two Minerals Never Before Seen on Earth
Meteorite That Recently Fell in Somalia Turns Out to Contain Two Minerals Never Before Seen on Earth  Nearly Frozen Waves Captured On Camera By Nantucket Photographer
Nearly Frozen Waves Captured On Camera By Nantucket Photographer  It’s Official: Astronomers Have Discovered another Earth
It’s Official: Astronomers Have Discovered another Earth 